A groundbreaking drug candidate, developed through the collaborative efforts of UCL and Moorfields Eye Hospital, has demonstrated high efficacy in treating a rare and potentially sight-threatening eye infection. The drug candidate, currently under development by SIFI S.p.A., has shown significant effectiveness in an international clinical trial.
The results of the study, published in Ophthalmology, highlight the efficacy and safety of the first-ever drug candidate for the treatment of Acanthamoeba keratitis (AK), employing a novel evidence-based treatment protocol.
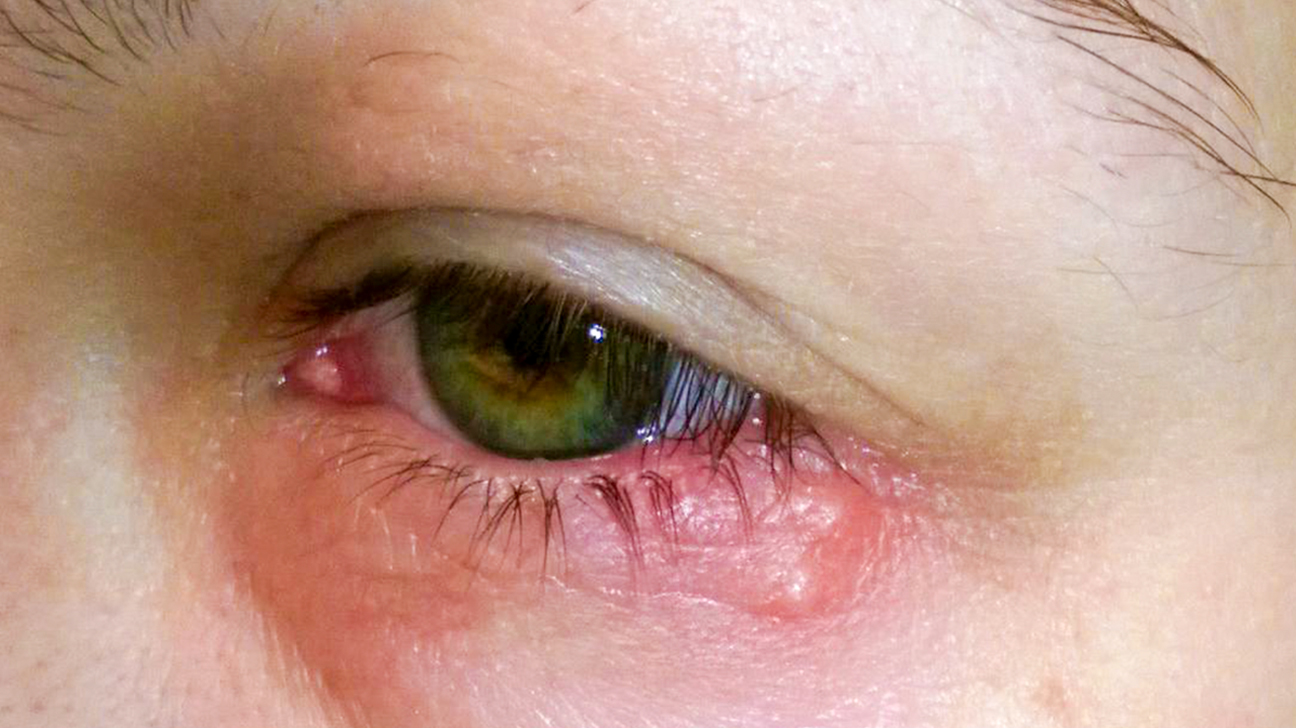
AK is a type of microbial keratitis, which is characterized by inflammation of the cornea, the clear protective outer layer of the eye. AK can cause severe pain and sensitivity to light.
Though relatively uncommon, AK affects approximately one in 37,000 contact lens wearers in the UK each year and accounts for approximately half of all cases of vision loss in this group. Individuals who wear reusable contact lenses are at higher risk of developing the disease, with a recent study by UCL and Moorfields revealing that the risk is nearly four times higher than for those wearing daily disposables. Additional risk factors include wearing lenses overnight and showering with lenses in.
The treatment being investigated, low concentration polihexanide (PHMB 0.02%), was initially compounded and used in the 1990s to treat AK. It was introduced by a team co-led by the lead author of the current study, Professor John Dart. While the treatment is widely recommended for AK, it is not a licensed drug, and treatment outcomes have varied.
The Phase 3 randomized controlled double-blind clinical trial followed a Phase 1 trial in healthy volunteers, which confirmed the safety of a higher concentration (0.08%) of polihexanide. The Phase 3 trial, conducted according to scientific advice from the European Medicines Agency, compared the effectiveness and safety of the high concentration polihexanide monotherapy to a commonly used dual therapy involving a lower dose of PHMB (0.02%) and propamidine. The Italian pharmaceutical company SIFI sponsored and funded all trials, with partial co-funding from the European Commission.
The study involved the evaluation of 127 individuals receiving treatment for AK across six hospitals in Europe, including England, Italy, and Poland.
The researchers found both formulations to be highly effective when used in conjunction with the prescribed drug delivery protocol, with an overall medical cure rate of 87%, meaning that 87% of patients were cured of AK without undergoing surgery. This is one of the highest cure rates reported for AK. The treatment failure rate was 13.4%, with almost half of the failures requiring therapeutic corneal transplant surgery. The overall corneal transplant surgery rate was one of the lowest ever reported for AK, at 6.3%.
The study authors attribute the higher effectiveness of the dual therapy to the strict adherence of clinicians to the treatment protocol. Additionally, the new monotherapy offers advantages over the dual therapy by simplifying the treatment process and reducing the risk of errors.
Dr. Vincenzo Papa, Head of Scientific Affairs at SIFI and co-author of the study, expressed the importance of the research, stating, “This publication in Ophthalmology, the leading peer-reviewed journal in our field, further encourages our continued efforts to make polihexanide 0.08% (Akantior®) available to patients with AK, as the first approved orphan medicinal product. Given the extreme burden of the disease and the high unmet medical need, we are proud of the high efficacy outcomes in the robust setting that the trial created, especially when compared with the efficacy rates of 60% reported with the current best treatment.”
SIFI has accumulated comprehensive quality, preclinical, and clinical data over 15 years of research and is now seeking regulatory approvals for polihexanide 0.08% in Europe, the UK, and the US.
Juliette Vila Sinclair Spence, Rare Disease Patient Advocate & Chairwoman of the Acanthamoeba Keratitis Eye Foundation, expressed excitement about the potential impact of the research, saying, “AK Warriors (aka patients) are now one step closer to receiving the first-ever product with a standardized protocol for Acanthamoeba keratitis. This is starting to bring light to the end of the tunnel.”
Acanthamoeba Keratitis is caused by the infection of Acanthamoeba, a cyst-forming microorganism, which leads to painful inflammation of the cornea. Severe cases of AK can result in vision loss or blindness, requiring prolonged treatment, and approximately 25% of those affected require corneal transplants.
Contact lens use has become the leading cause of microbial keratitis in individuals with otherwise healthy eyes in developed countries. While sight loss resulting from microbial keratitis is uncommon, Acanthamoeba is one of the most severe causes of the condition and is responsible for approximately half of all cases of vision loss in contact lens wearers. 90% of AK cases are attributed to avoidable risks. In recent years, the prevalence of AK has been on the rise in southeastern England, according to a study conducted by UCL and Moorfields. The findings of the study are also significant for populations in the global south, including parts of India, where agriculture-associated corneal trauma is the leading risk factor for AK.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it